CONVENIENT, ONCE-DAILY DOSING WITH ORGOVYX®1
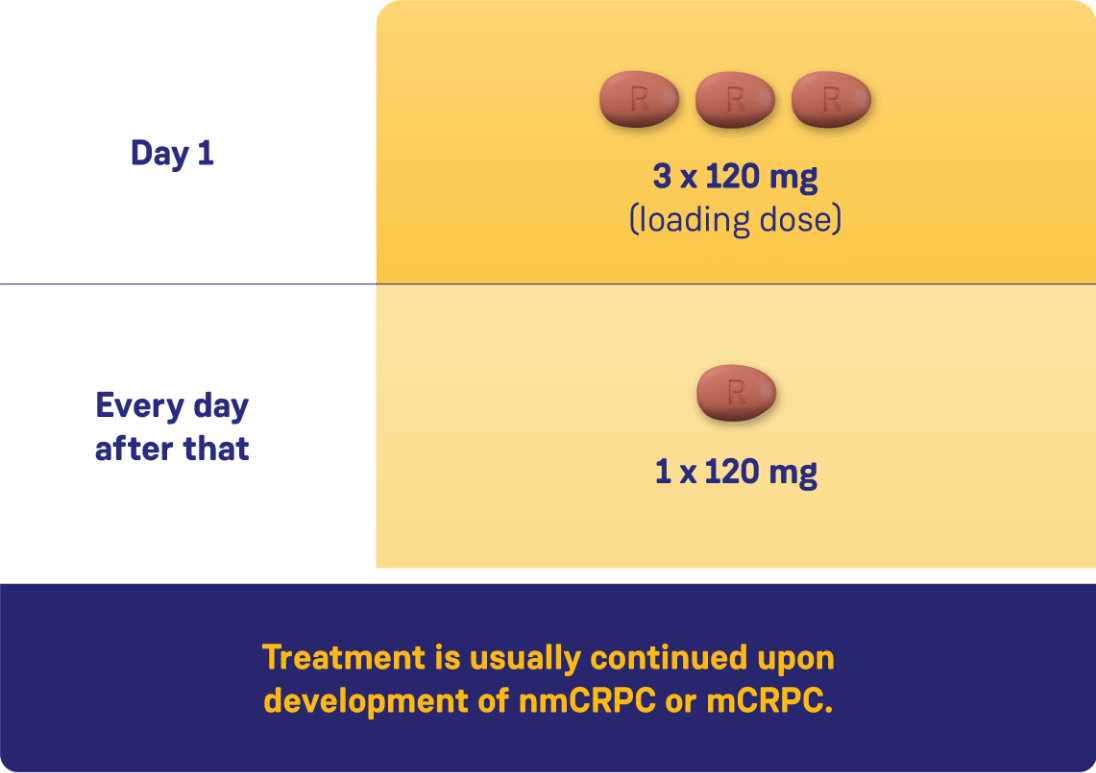
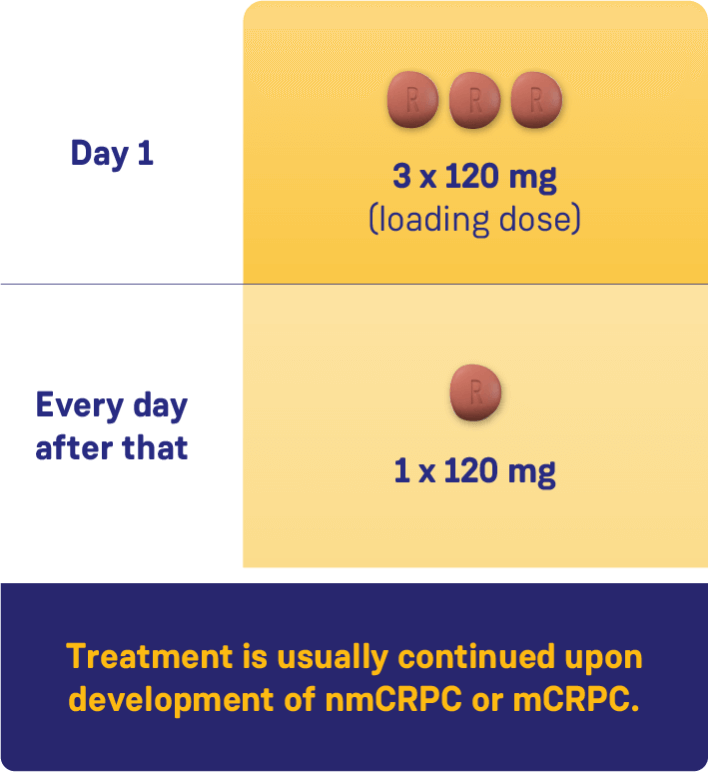
Adapted from the Product Monograph.1
nmCRPC: nonmetastatic castration-resistant prostate cancer;
mCRPC: metastatic castration-resistant prostate cancer
mCRPC: metastatic castration-resistant prostate cancer
- ORGOVYX® does not cause an increase in testosterone concentrations (clinical flare).
- Therefore, it is not necessary to add an anti-androgen as mitigation to avoid the clinical flare after initiation of therapy.
- ORGOVYX® can be taken with or without food.
- ORGOVYX® should be taken at approximately the same time each day.
- Instruct patients to swallow tablets whole and not to crush or chew tablets.
For complete dosing and administration instructions, please refer to the ORGOVYX® Product Monograph.
nmCRPC: nonmetastatic castration-resistant prostate cancer; mCRPC: metastatic castration-resistant prostate cancer
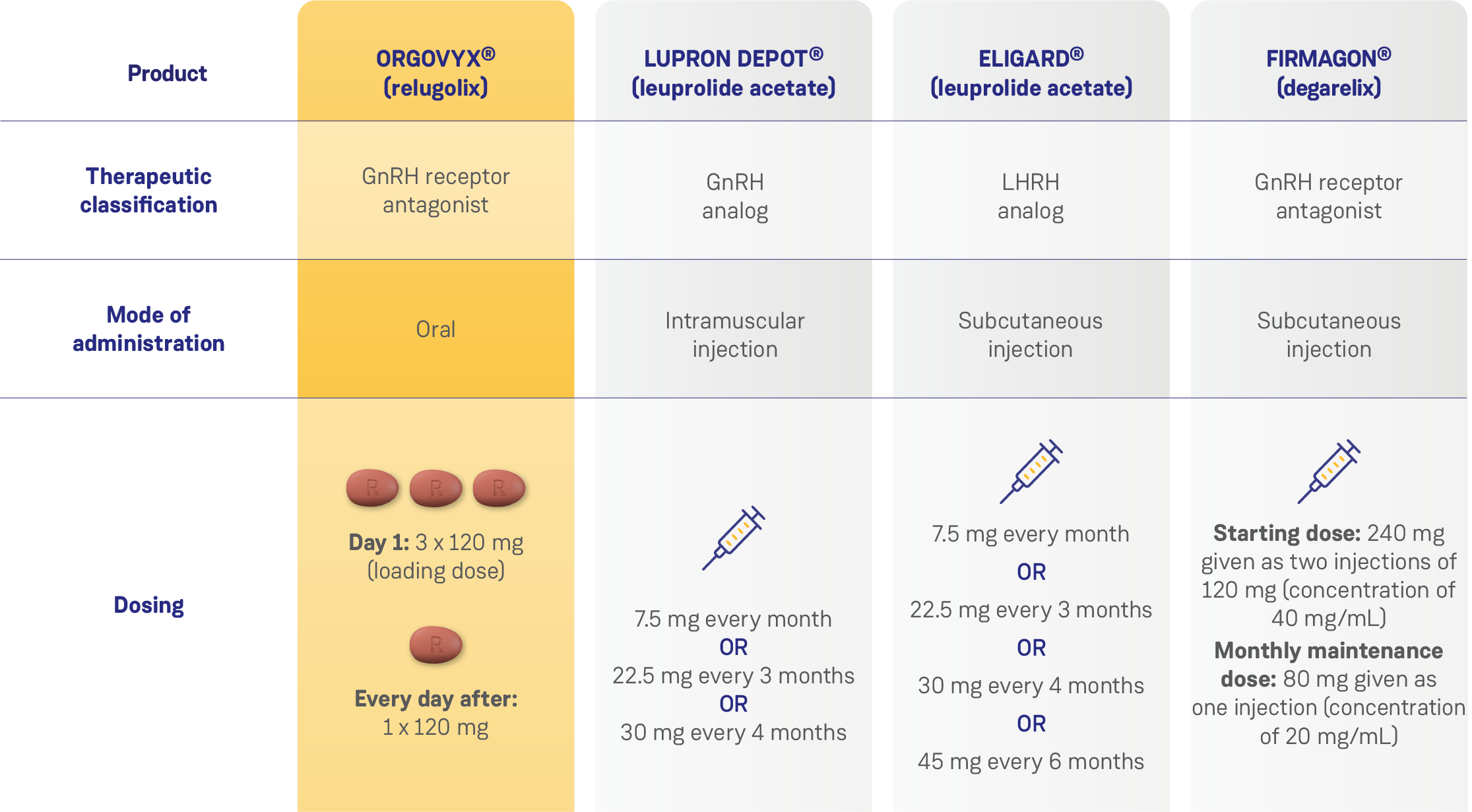
COMPARISON OF SELECT TREATMENT OPTIONS FOR ADVANCED PROSTATE CANCER1–4
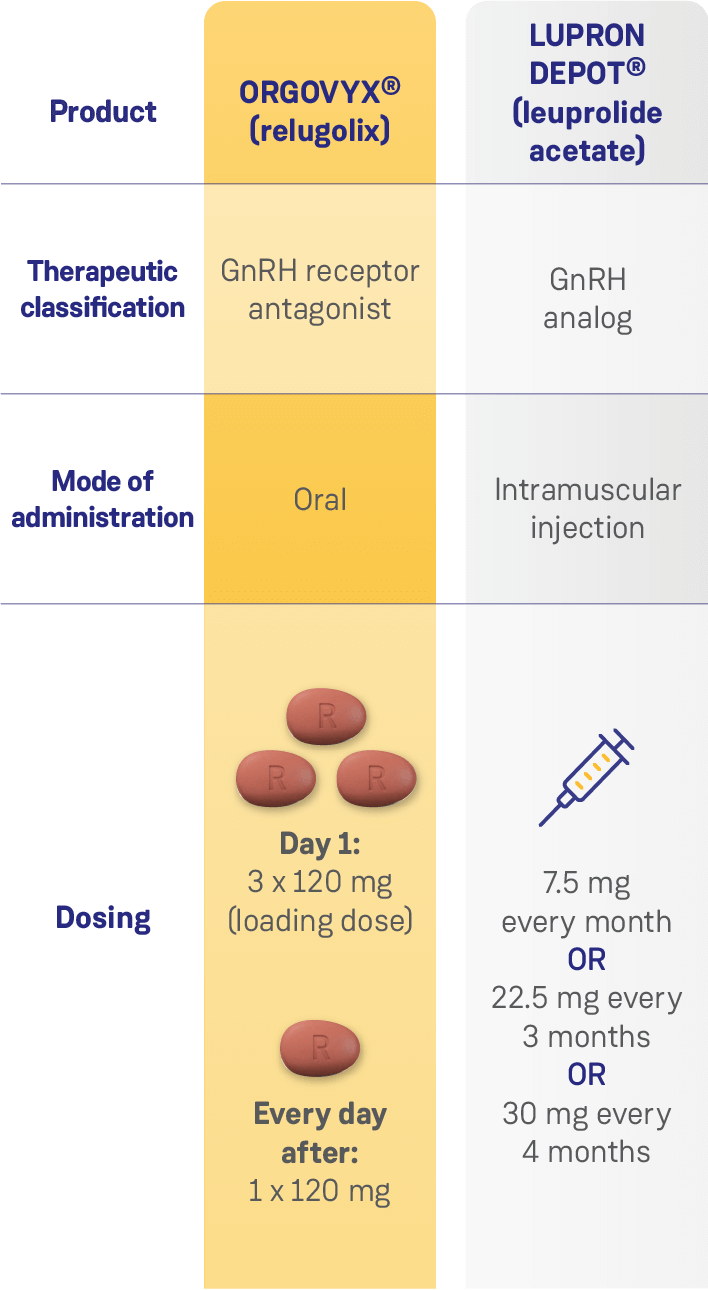
Data from separate product monographs; comparative clinical significance has not been proven.
Resources
Access relevant information and
additional resources for you
and your patients.
LHRH: luteinizing hormone-releasing hormone; GnRH: gonadotropin-releasing hormone
References: 1. ORGOVYX® Product Monograph. Sumitomo Pharma Switzerland GmbH. November 3, 2023.2. LUPRON® DEPOT Product Monograph. AbbVie Corporation. March 19, 2024.3. ELIGARD® Product Monograph. Tolmar International Ltd. January 26, 2024.4. FIRMAGON® Product Monograph. Ferring Pharmaceuticals. March 18, 2016.