EFFICACY DATA FROM
THE HERO STUDY
MEDICAL CASTRATION RATE (DEFINED AS ACHIEVING AND MAINTAINING TESTOSTERONE SUPPRESSION < 50 ng/dL) FROM DAY 29 THROUGH WEEK 48 (PRIMARY ENDPOINT)*,1
Mean percent change from baseline in testosterone concentration




Cumulative probability of testosterone suppression on Day 15*,1
Secondary endpoint
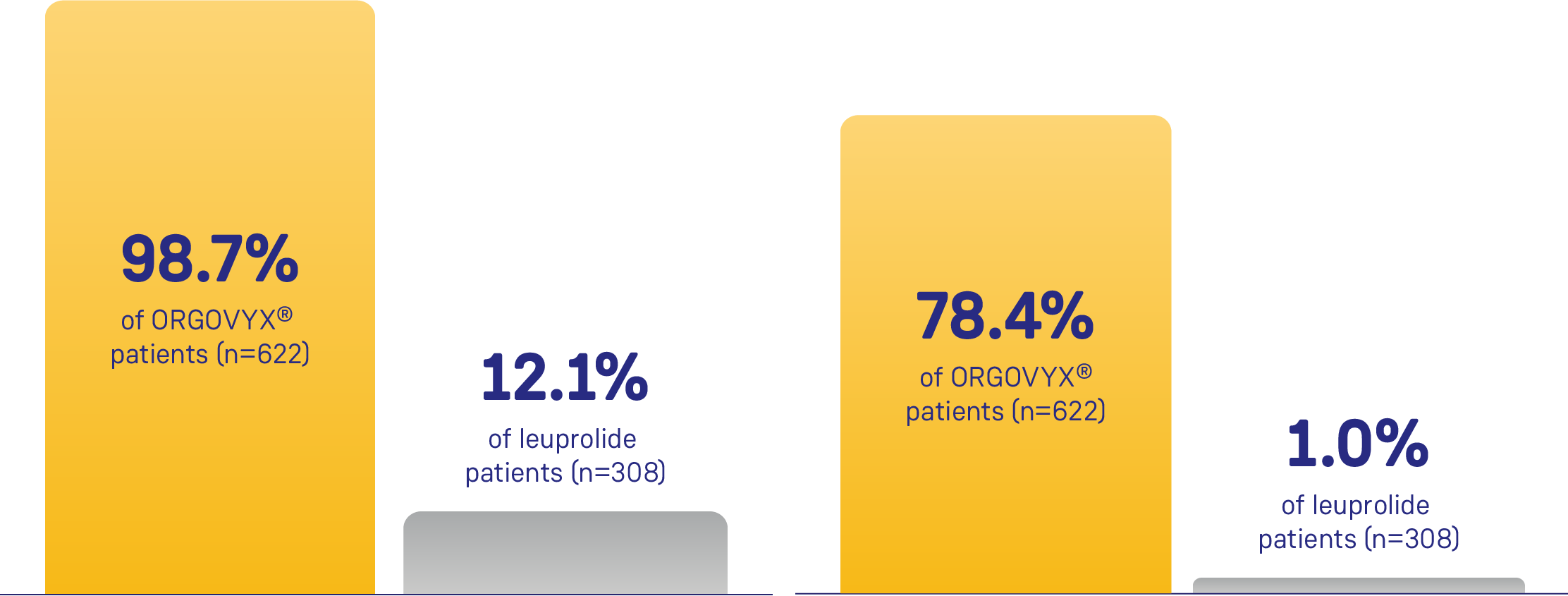
Testosterone suppression prior to Day 15 dose (< 50 ng/dL)

Testosterone suppression prior to Day 15 dose (< 20 ng/dL)

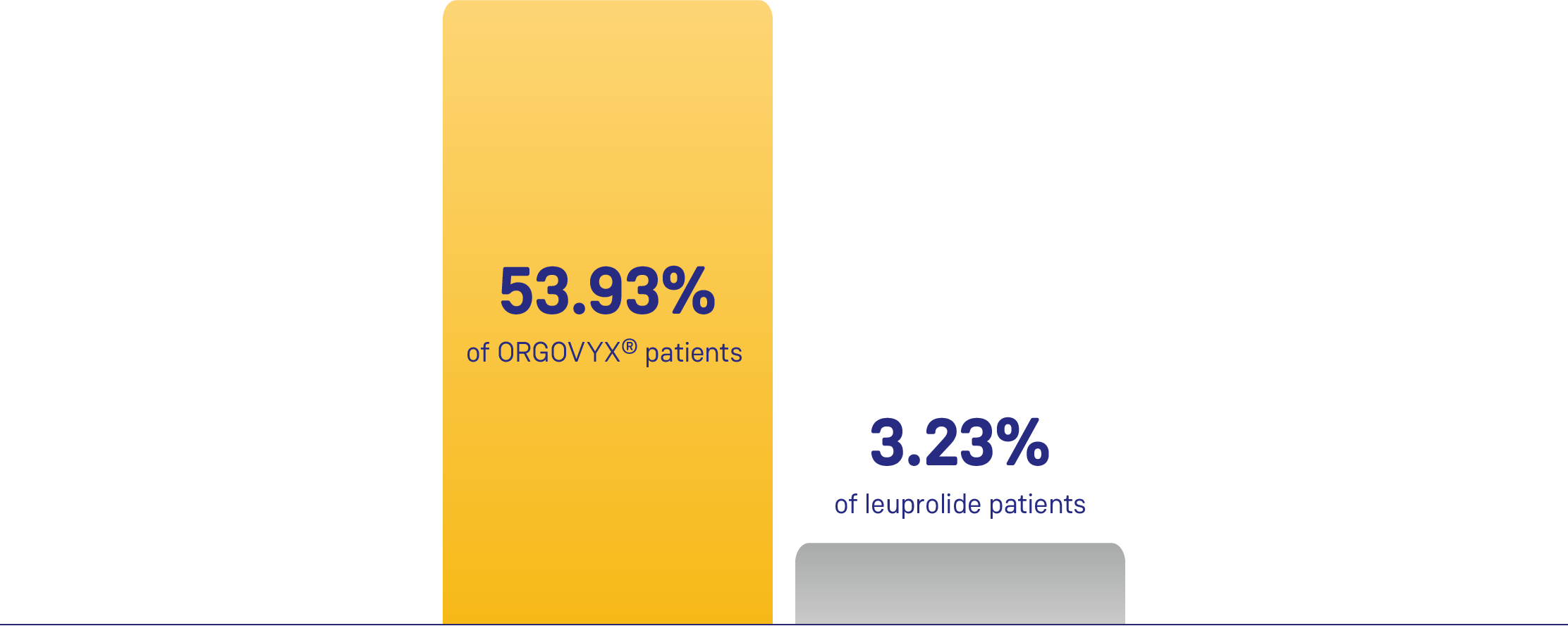
Descriptive Analysis: Cumulative
Probability of Testosterone Recovery (> 280 ng/dL) (N=184)*,1
Evaluated in a subset of patients who completed 48 weeks of treatment and were not offered alternative ADT upon completion for at least 90 days after discontinuation of either arm.

HERO STUDY
Learn more about the HERO study,
which evaluated ORGOVYX® in
advanced prostate cancer patients.
CI: confidence interval;
ADT: androgen deprivation therapy
*HERO: A Phase 3, multinational, randomized, open-label, parallel-group study comparing ORGOVYX® (n=622) to leuprolide (n=308) in adult men with androgen-sensitive advanced prostate cancer requiring at least 1 year of ADT. Patients were randomized 2:1 to receive ORGOVYX® (120 mg once daily after a single oral loading dose of 360 mg) or leuprolide acetate (22.5 mg [or 11.25 mg in Japan or Taiwan]) injection subcutaneously every 3 months. Leuprolide acetate 11.25 mg is a dosage regimen that is not recommended for this indication in Canada. The primary efficacy outcome measure was medical castration rate, defined as achieving and maintaining serum testosterone suppression to castrate levels (< 50 ng/dL) by Day 29 through 48 weeks of treatment.2
References: 1. ORGOVYX® Product Monograph. Sumitomo Pharma Switzerland GmbH. November 3, 2023. 2. Shore ND et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(32):2187–2196.
