Hero study design1,2
A Phase 3, multinational, randomized, open-label, parallel-group study evaluating the safety and efficacy profiles of ORGOVYX®.

Key Inclusion Criteria:
- Requiring at least 1 year of ADT with one of the following clinical disease presentations:
- Evidence of biochemical (PSA) or clinical relapse following local primary intervention
- Newly diagnosed androgen-sensitive metastatic disease
- Advanced localized disease unlikely to be cured by primary intervention with either surgery or radiation
- ECOG score 0/1
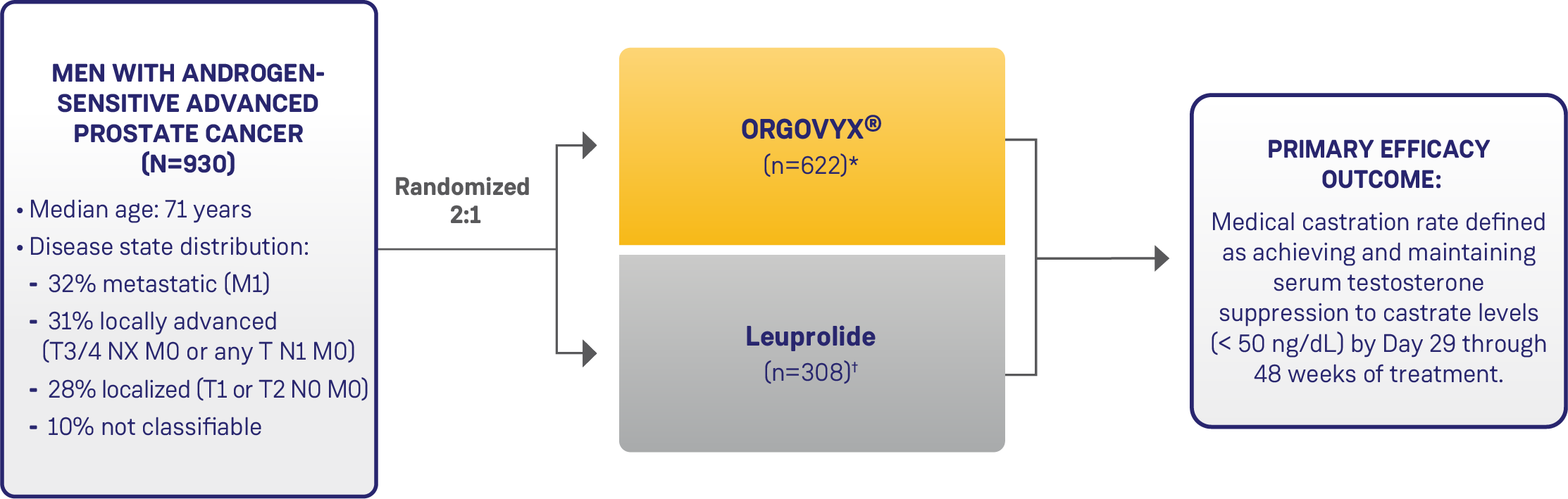

Adapted from the Product Monograph.1
EFFICACY PROFILE
Evaluate the ORGOVYX® efficacy data from the HERO study:
Testosterone suppression data (Day 29 to Week 48) Learn More
Testosterone suppression
data at Day 15 Learn More
data at Day 15 Learn More
Testosterone
recovery data Learn More
recovery data Learn More
- ADT: androgen deprivation therapy; ECOG: Eastern Cooperative Oncology Group
- *120 mg once daily after a single oral loading dose of 360 mg.
- †22.5 mg (or 11.25 mg in Japan or Taiwan) injection subcutaneously every 3 months. Leuprolide acetate 11.25 mg is a dosage regimen that is not recommended for this indication in Canada.2
References: 1. ORGOVYX® Product Monograph. Sumitomo Pharma Switzerland GmbH. November 3, 2023. 2. Shore ND et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(32):2187–2196.
